Thermodynamics:
The interactive Ellingham diagram
Here we shall go through the basic thermodynamics that lies behind use of the Ellingham diagram. First, we will establish the link between the thermodynamics of a reaction and its chemistry.
The Gibbs free energy, G, of a system can be described as the energy in the system available to do work. It is one of the most useful state functions in thermodynamics as it considers only variables contained within the system, at constant temperature and pressure.
It is defined as:
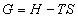
(1)
Here, T is the temperature of the system and S is the entropy, or disorder, of the system. H is the enthalpy of the system, defined as;
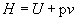
(2)
Where U is the internal energy, p is the pressure and v is the volume.
To see how the free energy changes when the system is changed by a small amount, we can differentiate the above functions:
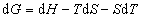
(3)
and;
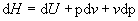
(4)
From the first law,
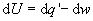
(5)
and from the second law,
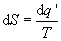
, (6)
we see that,
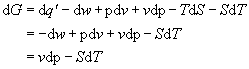
(7)
since work,
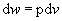
.
The above equation shows that if the temperature and pressure are kept constant, we see that the free energy does not change. This means that the Gibbs free energy of a system is unique at each temperature and pressure.
At a constant temperature dT = 0 and so
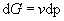
(8)
We can find G for the system by integration. To do this we need the system’s equation of state, to give a relationship between v and p.
We will consider an ideal gas. For one mole of an ideal gas the equation of state is;
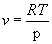
, (9)
so (8) becomes
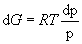
. (10)
Integrating:
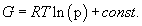
(11)
This we can express as
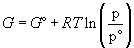
(12)
We define G° to be the standard free energy at the standard pressure, p°. These standard values are nothing more than lower integration constants, but using them is very useful, as we shall see. They are a consequence of the fact that one can only describe energy changes absolutely – there is no absolute energy scale, so the energy value we give to a system is arbitrary.
Chemical Reactions:
We will now see why studying the free energy of a system is useful in determining its behaviour.
The free energy change, ΔG, of a chemical reaction is the difference in free energy between the products of the reaction and the reactants. If the free energy of the products is less than the free energy of the reactants there will be a driving force for the reaction to occur.
For the reaction
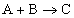
,
the free energy change,
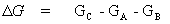
(13)
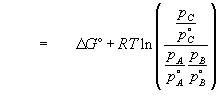
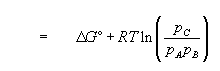
(14)
if the standard states
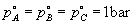
. We see that the free energy change of a reaction is determined by the relative quantities of reactants and products.
The Equilibrium Constant:
A chemical reaction will occur if the total free energy of the products is less than the total free energy of the reactants. (ie. The free energy change for the reaction is negative.) If the system containing the reactants and products is closed (if there is no input of reactants, for example), the concentration of reactants will decrease and the concentration of products will increase as the reaction proceeds. This will alter the state of the system and therefore alter the free energy change for the reaction (see equation 14, above).
The reaction will continue if the free energy change remains negative. Hence, the system proceeds down a free energy gradient with respect to composition and this gradient provides the driving force for the reaction to proceed. The system alters the quantities of reactants and products in response to the driving force until a minimum in free energy is reached and the gradient is zero. This is a point of equilibrium.
At equilibrium the free energy change for the reaction is equal to zero:
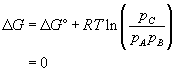
Therefore
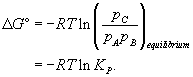
(15)
For the composition at equilibrium, the quotient is equal to
KP - the equilibrium constant for the reaction at constant pressure. We see that the equilibrium composition of the system is defined by the standard free energy change, Δ
G°. Equation 15 provides a link between the thermodynamics of a reaction and its chemistry. Δ
G° for a reaction is hence a very useful value to know.
Partial pressure of reacting gas:
Using equations (15) we can see that the equilibrium constant is related to the partial pressures of reacting gases:
for the reaction
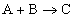
. (Remember that these pressures must be related to a standard state.)
For a metal oxidation reaction ,
2M (s) + O2 (g) = 2MO (s) ,
the equilibrium constant has the form
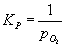
.
We can therefore find the equilibrium partial pressure of oxygen at a particular temperature from the value of ΔG°:
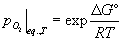
.
The equilibrium partial pressure of oxygen is the pressure at which the driving force for the reaction is zero. From equation 14 we see that if the partial pressure of oxygen is greater than this value, the free energy change for the reaction is negative and there is a driving force for the reaction to take place. Metal will be oxidised, and the partial pressure of oxygen will drop until it reaches equilibrium. This is effect described by Le Chatelier’s principle.
If the partial pressure of oxygen is below the equilibrium value, oxidation is avoided. (In fact, the metal oxide will disassociate to form metal plus oxygen gas-this is because there is a driving force for the reaction to proceed backward. For this reason the equilibrium partial pressure is often known as the dissociation pressure.)
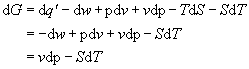 (7)
(7)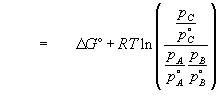
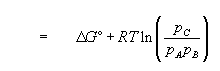 (14)
(14)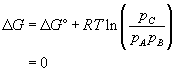
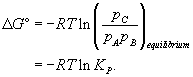 (15)
(15)