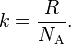I had read some theoretical paper about defect in GaN. They analysis the defect using formation energies as a function of Fermi level. If the formation energy is too big, this defect is very unlikely to be seen. So what does this “formation energy” mean?
The formation energy is the energy required to produce a defect (a vacancy) into the perfect crystal structure.In the case of GaN, the formation energy of each defect is a function of the Fermi energy and the difference of the chemical potentials between Ga and N.
The formation energy is the cost of creating a defect into an otherwise perfect solid. For a vacancy it is calculated as the energy needed to remove an atom from the bulk and take it to infinity. Formation energies are very important because the concentration of defects in a solid in thermodynamic equilibrium depends exponentially on it.
The formation energy is the energy required to produce a defect (a vacancy) into the perfect crystal structure.In the case of GaN, the formation energy of each defect is a function of the Fermi energy and the difference of the chemical potentials between Ga and N.
The formation energy is the cost of creating a defect into an otherwise perfect solid. For a vacancy it is calculated as the energy needed to remove an atom from the bulk and take it to infinity. Formation energies are very important because the concentration of defects in a solid in thermodynamic equilibrium depends exponentially on it.
What's the differences among the concepts: binding energy, cohesive energy and formation energy?
- Formation energy is the change in energy when a material is formed from it's constituent elements in their reference states. For example, the formation energy of alumina (Al2O3) is the change in energy when fcc aluminum and O2 gas combine to make Al2O3.
- Cohesive energy is the amount of energy it takes to break something up into isolated atoms. This is also called the atomization energy.
- Binding energy in general means the amount of energy to split something up, and can mean different things depending on the context. For example, if you're talking about a molecule, it can refer to atomization energy.
Something to keep in mind regarding sign conventions is that formation energies are typically given as negative values, whereas cohesive energies and binding energies are typically positive values.